Rates of Heat Transfer
Rates of Heat Transfer
On previous pages of this lesson, we have learned that heat is a form of energy transfer from a high temperature location to a low temperature location. The three main methods of heat transfer - conduction, convection and radiation - were discussed in detail on the previous page. Now we will investigate the topic of the rate of heat transfer. This topic is of great importance because of the frequent need to either increase or decrease the rate at which heat flows between two locations. For instance, those of us who live in colder winter climates are in constant pursuit of methods of keeping our homes warm without spending too much money. Heat escapes from higher temperature homes to the lower temperature outdoors through walls, ceilings, windows and doors. We make efforts to reduce this heat loss by adding better insulation to walls and attics, caulking windows and doors, and buying high efficiency windows and doors. As another example, consider electricity generation. Household electricity is most frequently manufactured by using fossil fuels or nuclear fuels. The method involves generating heat in a reactor. The heat is transferred to water and the water carries the heat to a steam turbine (or other type of electrical generator) where the electricity is produced. The challenge is to efficiently transfer the heat to the water and to the steam turbine with as little loss as possible. Attention must be given to increasing heat transfer rates in the reactor and in the turbine and decreasing heat transfer rates in the pipes between the reactor and the turbine.
So what variables would affect the heat transfer rates? How can the rate of heat transfer be controlled? These are the questions to be discussed on this page of Lesson 1. Our discussion will be restricted to the variables affecting the rate of heat transfer by conduction. Once the variables affecting the rate of heat transfer are discussed, we will look at a mathematical equation that expresses the dependence of rate upon these variables.
Temperature Difference
 In conduction, heat is transferred from a hot temperature location to a cold temperature location. The transfer of heat will continue as long as there is a difference in temperature between the two locations. Once the two locations have reached the same temperature, thermal equilibrium is established and the heat transfer stops. Earlier in this lesson, we discussed the transfer of heat for a situation involving a metal can containing high temperature water that was placed within a Styrofoam cup containing low temperature water. If the two water samples are equipped with temperature probes that record changes in temperature with respect to time, then the following graphs are produced.
In conduction, heat is transferred from a hot temperature location to a cold temperature location. The transfer of heat will continue as long as there is a difference in temperature between the two locations. Once the two locations have reached the same temperature, thermal equilibrium is established and the heat transfer stops. Earlier in this lesson, we discussed the transfer of heat for a situation involving a metal can containing high temperature water that was placed within a Styrofoam cup containing low temperature water. If the two water samples are equipped with temperature probes that record changes in temperature with respect to time, then the following graphs are produced.
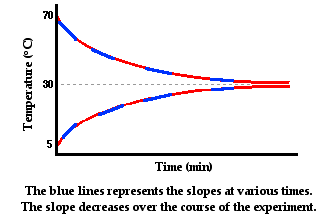
In the graphs above, the slope of the line represents the rate at which the temperature of each individual sample of water is changing. The temperature is changing because of the heat transfer from the hot to the cold water. The hot water is losing energy, so its slope is negative. The cold water is gaining energy, so its slope is positive. The rate at which temperature changes is proportional to the rate at which heat is transferred. The temperature of a sample changes more rapidly if heat is transferred at a high rate and less rapidly if heat is transferred at a low rate. When the two samples reach thermal equilibrium, there is no more heat transfer and the slope is zero. So we can think of the slopes as being a measure of the rate of heat transfer. Over the course of time, the rate of heat transfer is decreasing. Initially heat is being transferred at a high rate as reflected by the steeper slopes. And as time progresses, the slopes of the lines are becoming less steep and more gently sloped.
What variable contributes to this decrease in the heat transfer rate over the course of time? Answer: the difference in temperature between the two containers of water. Initially, when the rate of heat transfer is high, the hot water has a temperature of 70°C and the cold water has a temperature of 5°C. The two containers have a 65°C difference in temperature. As the hot water begins to cool and the cold water begins to warm, the difference in their temperatures decrease and the rate of heat transfer decreases. As thermal equilibrium is approached, their temperatures are approaching the same value. With the temperature difference approaching zero, the rate of heat transfer approaches zero. In conclusion, the rate of conductive heat transfer between two locations is affected by the temperature difference between the two locations.
Material
The first variable that we have identified as affecting the rate of conductive heat transfer is the temperature difference between the two locations. The second variable of importance is the materials involved in the transfer. In the previous discussed scenario, a metal can containing high temperature water was placed within a Styrofoam cup containing low temperature water. The heat was transferred from water through the metal to water. The materials of importance were water, metal and water. What would happen if the heat were transferred from hot water through glass to cold water? What would happen if the heat were transferred from hot water through Styrofoam to cold water? Answer: the rate of heat transfer would be different. Replacing the inner metal can with a glass jar or a Styrofoam cup would change the rate of heat transfer. The rate of heat transfer depends on the material through which heat is transferred.
The effect of a material upon heat transfer rates is often expressed in terms of a number known as the thermal conductivity. Thermal conductivity values are numerical values that are determined by experiment. The higher that the value is for a particular material, the more rapidly that heat will be transferred through that material. Materials with relatively high thermal conductivities are referred to as thermal conductors. Materials with relatively low thermal conductivity values are referred to as thermal insulators. The table below lists thermal conductivity values (k) for a variety of materials, in units of W/m/°C.
|
Material |
k |
|
Material |
k |
|
|
Aluminum (s) |
237 |
|
Sand (s) |
0.06 |
|
|
Brass (s) |
110 |
|
Cellulose (s) |
0.039 |
|
|
Copper (s) |
398 |
|
Glass wool (s) |
0.040 |
|
|
Gold (s) |
315 |
|
Cotton wool (s) |
0.029 |
|
|
Cast Iron (s) |
55 |
|
Sheep's wool (s) |
0.038 |
|
|
Lead (s) |
35.2 |
|
Cellulose (s) |
0.039 |
|
|
Silver (s) |
427 |
|
Expanded Polystyrene (s) |
0.03 |
|
|
Zinc (s) |
113 |
|
Wood (s) |
0.13 |
|
|
Polyethylene (HDPE) (s) |
0.5 |
|
Acetone (l) |
0.16 |
|
|
Polyvinyl chloride (PVC) (s) |
0.19 |
|
Water (l) |
0.58 |
|
|
Dense Brick (s) |
1.6 |
|
Air (g) |
0.024 |
|
|
Concrete (Low Density) (s) |
0.2 |
|
Argon (g) |
0.016 |
|
|
Concrete (High Density) (s) |
1.5 |
|
Helium (g) |
0.142 |
|
|
Ice (s) |
2.18 |
|
Oxygen (g) |
0.024 |
|
|
Porcelain (s) |
1.05 |
|
Nitrogen (g) |
0.024 |
Source: http://www.roymech.co.uk/Related/Thermos/Thermos_HeatTransfer.html
As is apparent from the table, heat is generally transferred by conduction at considerably higher rates through solids (s) in comparison to liquids (l) and gases (g). Heat transfer occurs at the highest rates for metals (first eight items in left-hand column) because the mechanism of conduction includes mobile electrons (as discussed on a previous page). Several of the solids in the right-hand column have very low thermal conductivity values and are considered insulators. The structure of these solids is characterized by pockets of trapped air interspersed between fibers of the solid. Since air is a great insulator, the pockets of air interspersed between these solid fibers gives these solids low thermal conductivity values. One of these solid insulators is expanded polystyrene, the material used in Styrofoam products. Such Styrofoam products are made by blowing an inert gas at high pressure into the polystyrene before being injected into the mold. The gas causes the polystyrene to expand, leaving air filled pockets that contribute to the insulating ability of the finished product. Styrofoam is used in coolers, pop can insulators, thermos jugs, and even foam boards for household insulation. Another solid insulator is cellulose. Cellulose insulation is used to insulate attics and walls in homes. It insulates homes from heat loss as well as sound penetration. It is often blown into attics as loose fill cellulose insulation. It is also applied as fiberglass batts (long sheets of paper backed insulation) to fill the spacing between 2x4 studs of the exterior (and sometimes interior) walls of homes.
Area
 Another variable that affects the rate of conductive heat transfer is the area through which heat is being transferred. For instance, heat transfer through windows of homes is dependent upon the size of the window. More heat will be lost from a home through a larger window than through a smaller window of the same composition and thickness. More heat will be lost from a home through a larger roof than through a smaller roof with the same insulation characteristics. Each individual particle on the surface of an object is involved in the heat conduction process. An object with a wider area has more surface particles working to conduct heat. As such, the rate of heat transfer is directly proportional to the surface area through which the heat is being conducted.
Another variable that affects the rate of conductive heat transfer is the area through which heat is being transferred. For instance, heat transfer through windows of homes is dependent upon the size of the window. More heat will be lost from a home through a larger window than through a smaller window of the same composition and thickness. More heat will be lost from a home through a larger roof than through a smaller roof with the same insulation characteristics. Each individual particle on the surface of an object is involved in the heat conduction process. An object with a wider area has more surface particles working to conduct heat. As such, the rate of heat transfer is directly proportional to the surface area through which the heat is being conducted.
Thickness or Distance
A final variable that affects the rate of conductive heat transfer is the distance that the heat must be conducted. Heat escaping through a Styrofoam cup will escape more rapidly through a thin-walled cup than through a thick-walled cup. The rate of heat transfer is inversely proportional to the thickness of the cup. A similar statement can be made for heat being conducted through a layer of cellulose insulation in the wall of a home. The thicker that the insulation is, the lower the rate of heat transfer. Those of us who live in colder winter climates know this principle quite well. We are told to dress in layers before going outside. This increases the thickness of the materials through which heat is transferred, as well as trapping pockets of air (with high insulation ability) between the individual layers.
A Mathematical Equation
So far we have learned of four variables that affect the rate of heat transfer between two locations. The variables are the temperature difference between the two locations, the material present between the two locations, the area through which the heat will be transferred, and the distance it must be transferred. As is often the case in physics, the mathematical relationship between these variables and the rate of heat transfer can be expressed in the form of an equation. Let's consider the transfer of heat through a glass window from the inside of a home with a temperature of T1 to the outside of a home with a temperature of T2. The window has a surface area A and a thickness d. The thermal conductivity value of the window glass is k. The equation relating the heat transfer rate to these variables is
Rate = k•A•(T1 - T2)/d
The units on the rate of heat transfer are Joule/second, also known as a Watt. This equation is applicable to any situation in which heat is transferred in the same direction across a flat rectangular wall. It applies to conduction through windows, flat walls, slopes roofs (without any curvature), etc. A slightly different equation applies to conduction through curved walls such as the walls of cans, cups, glasses and pipes. We will not discuss that equation here.
Example Problem
To solve this problem, we will need to know the surface area of the window. Being a rectangle, we can calculate the area as width • height.
Area = (1.2 m)•(1.8 m) = 2.16 m2.
We will also need to give attention to the unit on thickness (d). It is given in units of cm; we will need to convert to units of meters in order for the units to be consistent with that of k and A.
d = 6.2 mm = 0.0062 m
Now we are ready to calculate the rate of heat transfer by substitution of known values into the above equation.
Rate = (0.27 W/m/°C)•(2.16 m2)•(21°C - -4°C)/(0.0062 m)
Rate = 2400 W (rounded from 2352 W)
It is useful to note that the thermal conductivity value of a house window is much lower than the thermal conductivity value of glass itself. The thermal conductivity of glass is about 0.96 W/m/°C. Glass windows are constructed as double and triple pane windows with a low pressure inert gas layer between the panes. Furthermore, coatings are placed on the windows to improve efficiency. The result is that there are a series of substances through which heat must consecutively pass in order to be transferred out of (or into) the house. Like electrical resistors placed in series, a series of thermal insulators has an additive effect on the overall resistance offered to the flow of heat. The accumulative effect of the various layers of materials in a window leads to an overall conductivity that is much less than a single pane of uncoated glass.
Lesson 1 of this Thermal Physics chapter has focused on the meaning of temperature and heat. Emphasis has been given to the development of a particle model of materials that is capable of explaining the macroscopic observations. Efforts have been made to develop solid conceptual understandings of the topic in the absence of mathematical formulas. This solid conceptual understanding will serve you well as you approach Lesson 2. The chapter will turn slightly more mathematical as we investigate the question: how can the amount of heat released from or gained by a system be measured? Lesson 2 will pertain to the science of calorimetry.
